Atom Frenzy – Lesson 7 – Runny and Sticky
Students explore the viscosity (or ‘runniness’ and ‘stickiness’) of common kitchen ingredients by observing how easily they pour from one container to another. They also develop a scale and use it to measure the ‘runniness’ and ‘stickiness’ of these liquids.
1. Introduction: Introduce Lesson 7 by indicating students will examine the ‘runniness’ or viscosity of some liquids and learn how the forces between the molecules in each liquid affect how easily they flow and how sticky they are. Then introduce the Lesson 7 learning intentions using the Atom Frenzy Lesson 7 PowerPoint.
2. Discussion – What is viscosity? The teacher introduces viscosity by relating to pouring honey or maple syrup on toast or pancakes, connecting it to how ‘runny’ or ‘sticky’ a liquid is and developing a scale to ‘measure’ viscosity. They then discuss how to measure viscosity of different liquid foods and use an investigation planning sheet to help ensure a ‘fair test’ in each case.
3. Activity (small groups) – The last drop (Method 1): Exploring runniness and stickiness: Working in groups, students test five different liquids. They pour a set volume (e.g. 10 mL) of each liquid kitchen ingredient or food from a small plastic container into a larger cup, measuring the time it takes to flow into the empty cup and rating its flow rate using the scale developed.
4. Activity (small groups) – Runny Races (Method 2): Alternatively, they may use a small plastic board with depressions cut out of one end to place the five liquids being tested. Each depression is filled with a liquid, the board is tipped so it is almost vertical, and the time it takes for each liquid to run down the length of the board is recorded.
5. Activity (whole class) – Molecules getting through a gap: In an open space, students time how long it takes for the whole class to fit through a small gap. Discuss and compare to how molecules move past each other and how it affects viscosity.
6. Discussion – Summary of concepts: Review findings of the investigations: the flow rate of different liquids at room temperature. Then, through questioning, ask students to describe their observations and explain why different liquids flow at different rates using the molecular model. Encourage students to relate explanations to the strength of the electrical forces between the molecules and the size of the molecules.
7. Review and introduce the next lesson: Review the main things learnt about viscosity including an explanation of different flow rates and new words and write them on the class Word Wall.
Students will:
- know that viscosity is a measure of the ‘runniness’ of a liquid
- with guidance, plan and conduct a scientific investigation to explore viscosity
- explain that the viscosity of liquids is affected by the forces holding the molecules together, the temperature and the size of the molecules.
- Lessons 7 and 8 provide an opportunity for students to explore the viscosity or ‘runniness and stickiness’ of common foods.
- The Lesson 7 activity is quite straight-forward and requires the students to work in groups. They pour liquids of different viscosity from a small plastic measuring cup to a larger collection cup and time how long each liquid takes to flow from one plastic glass to the other. An alternative activity is provided in which students allow liquids of different viscosity to run down a vertical piece of plastic.
- The Lesson 8 activity takes this further and explores how temperature affects viscosity using the same methods. It is advised that the teacher try both activities out and decide whether they consider their class could manage this. If not, it could be completed as a demonstration. If students’ investigation skills are sufficiently developed, it could also be used as an assessment.
- Purchase the different liquids from a supermarket.
- Purchase squeezy containers for those liquids not supplied in such a container.
- Obtain small, clear 30 mL plastic measuring cups.
- honey, golden syrup, maple syrup, cooking oil, and glucose syrup (or alternatives with a range of viscosities) each in a labelled squeezy bottle
- small, graduated plastic medicine glasses (30 mL) or plastic teaspoons
- alternatively, small waterproof boards, such as personal whiteboards
- timers
In this lesson we will examine the viscosity of liquids and learn how the forces between the molecules that are in each liquid affect how easily each liquids flows and how sticky it is.
Introduce the learning intentions for this lesson from the Atom Frenzy Lesson 7 PowerPoint.
- Who has tried to pour honey or maple syrup onto their toast or pancakes on a cold winter morning?
- What do you notice?
How hard it is for a liquid to flow is called viscosity. Viscosity is a new word to remember. A liquid like water flows easily and has low viscosity, whereas a liquid like honey that does not want to flow has high viscosity. Viscosity is caused by the internal friction between the molecules that make up the liquid. Viscosity is also related to how sticky something is. Most things that have a high viscosity are also very sticky.
In this lesson’s activity students will investigate the viscosity of different liquids. To do this we will need to be able to ‘measure’ how runny or sticky each liquid is.
- Can anyone suggest how we might measure viscosity? Students may suggest a range of approaches – explore each idea presented.
- Did anyone think of making our own scale that will allow us to describe how easily each liquid flows?
- How easily does water flow? Very easily.
So, tell students that we’ll make that the first point on our scale.
- Now what about a liquid that doesn’t flow very easily? Thick honey, treacle, etc.
Suggest to students we could develop our scale, something like:
-
- flows very easily (like water)
- flows easily (but not quite as runny as water)
- flows slowly
- flows very slowly
- hardly flows at all
This could be the student’s ‘viscosity scale’ that they will use to report their results.
Discuss how they can measure viscosity so they can be sure they have a ‘fair test’ in each case. Use the investigation planning sheet (Worksheet – Investigating Runniness).
Finally, ask students about how they can present their results. (Use a table.)
The planning phase could be completed as a class activity using the whiteboard, or students could complete the attached template in their groups prior to starting the activity.
Remind students of the simple rules: don’t make a mess; clean up any spills with warm soapy water and rag, or ask for help; never taste liquids in science – we are not sure whether they are contaminated etc.
We recommend viewing the first part of the activity video on the right before reading the detailed activity instructions below.
Set up five workstations each with one of five different liquids (if there are 10 groups, you could have two sets of stations) You may need to move between the work stations to explain.
At each station, place small plastic measuring cups (one small one per group and an empty larger one to pour the liquid into).
Liquids could include, but vary to suit availability:
- honey
- golden syrup
- maple syrup
- cooking oil
- glucose syrup
Ensure that all of the small plastic measuring cups have the same amount of liquid in each.
The larger, empty plastic cup is to pour the liquid into to collect for re-use by another class or next year.
Make sure each group follows the plan set out in their investigation planner and that each group has appointed a ‘timer’, a ‘pourer’ and a ‘recorder’ as well as the ‘director’.
In their groups, students work their way around each desk and test out how quickly (in seconds) and easily each liquid can be poured into an empty cup.
Students time how long it takes to pour each liquid from the plastic measuring cup to the collecting cup – until the last drop leaves the measuring cup – and judge how easily each liquid flows from the small plastic measuring cup to the container.
They record the time taken and how easily each liquid flowed using the scale developed earlier.
If students are familiar with the time-lapse video function on a tablet, then they could use these to time each liquid.
They should then use the table prepared for them on the worksheet.
When each of the five liquids has been tested and the results have been added to the results table, all used empty plastic measuring cups should be placed in a bucket of warm soapy water.
If time permits, and students have the graphing skills, the data could be presented using a simple column or dot graph.
We recommend viewing the second part of the activity video on the right before reading the detailed activity instructions that below.
This method uses a plastic board that has five holes drilled at one end as shown in the photograph. Alternatively you can use small portable whiteboards or similar with two lines marked at the top and bottom, as shown in the video.
Each of the small holes is filled with liquids (or place five dollops at the top of the board) such as:

- honey
- golden syrup
- maple syrup
- cooking oil
- glucose syrup
When the board has all holes filled with different liquids and all team members are clear about what each one will do (one to stand the board upright by leaning it on a small box, another to be the observer and recorder, a third member could be the timer of how long it takes each liquid to reach the bottom and a fourth member could film the activity using a tablet on Slo-Mo mode etc.), then carry out the investigation.
In their groups students:
- measure the time it takes for each liquid to reach the bottom of the board.
- record how easily each liquid flowed using our scale developed earlier.
- should use a tablet time-lapse video function if they are available and the students are familiar with it.
Show students the images of the traffic on a 30-lane highway in China getting through a road checkpoint, the sand in an egg timer, and football fans leaving a packed Optus Stadium at the end of a football match.
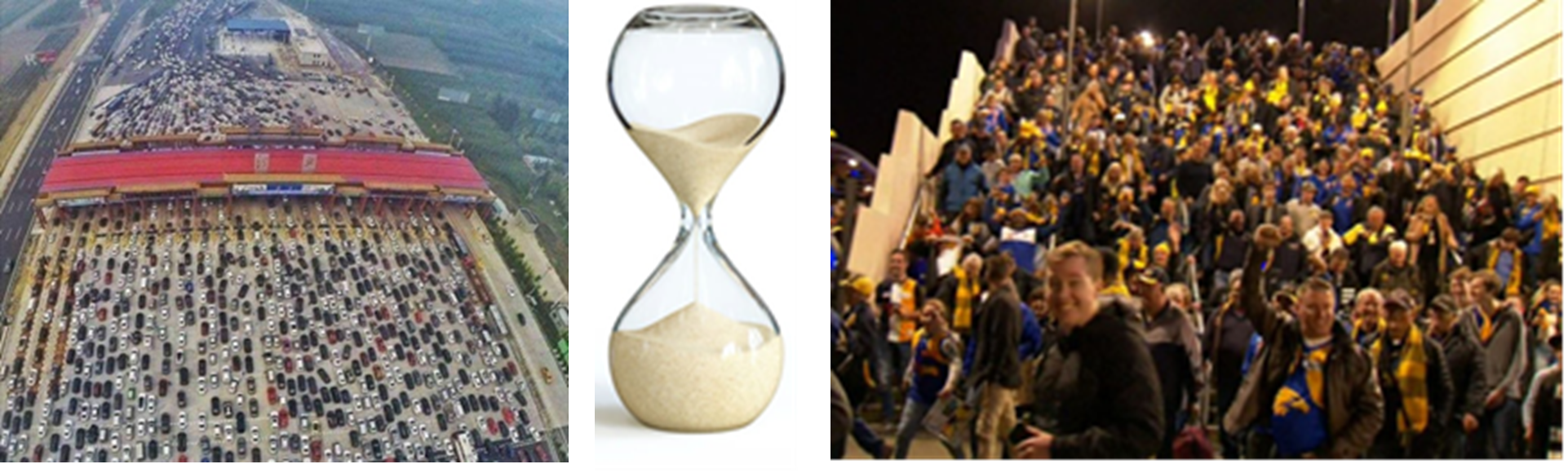
- What do these three pictures all have in common? Cars, people and grains of sand need to all get through a narrow gap.
- Do the cars, sand grains and people slow down when they go through a smaller gap? Yes, bouncing off each other and even just squeezing together slows things down quite a lot.
Go to a clear area outside your room – a wide passage will be OK, or right outside to a bitumen surface like a netball court. Measure out a distance of about ten metres. (~10 teacher steps) Note: A netball court is ideal because each third is about 10 m long.
- How long does it take our class to all walk the 10-metre distance? (Someone time using a timer.)
Let students know they will now be like cars in a traffic jam, people leaving a packed stadium or grains of sand in an egg timer. Use desks or chairs to make a narrow gap about half-way along the 10-metre distance. The gap should be about half a metre wide, and everyone must go through it.

Now how long does it take the class to all walk the 10-metre distance? Remember, they must go through the gap. Ask a student to time using a timer.
- Why did it take longer when we had to move through the gap? We were slowed down because we had to come close together and ‘merge’ to fit through the gap.
For real molecules, especially for big ones (like long strings of the magnetic marbles), it takes a while for them to jiggle past each other and get through the gap. This is due both to the electrical forces in between the molecules, and the physical size and shape of the molecules.
Students saw how different liquids flow. Some flowed easily, like water, and others flowed very slowly, like glucose syrup.
Let’s now review the findings for our five different liquids at room temperature:
- What do we mean when we say we want to rank something? Put them in order, from longest to shortest, or in today’s activity, runniest to stickiest.
- Ask one group’s reporter to tell the class their ranking: from flows very fast to hardly flows at all (Order expected is: cooking oil; maple syrup; honey; golden syrup and glucose syrup there may be variations due to the different constitution of some of the liquids and the temperature of the room.)
- How did other group’s list compare with this group’s ranking? Would we expect the order to be the same? Take time to discuss and explain differences – even invite some groups to re-try two liquids to see if they obtain a different result.
- What are all of these liquids made from? Most of them are a mixture of different molecules of different sizes, like oils, different types of sugar, some water and lots of other yummy molecules that we can eat and are good for us, provided we don’t eat too much of them of course!
- Why do you think some liquids flow very easily while others hardly flow at all? The strength of the forces between the different molecules – strong forces slow down or stop the molecules flowing over one another.
- What is friction – you may have learnt about it in science last year or from discussions at home? Friction is the force that slows things down or stops things moving and is sometimes called ‘drag’.
- What causes the friction or ‘drag’ between the molecules? The electrical forces between the neighbouring molecules.
Finally, students return to their planning sheet and spend a few minutes using what we have just learnt about viscosity and friction between molecules to explain why some of our liquids flow faster than others.
What were the main things we learnt today:
- What is viscosity? A measure of how much a liquid does not want to, or resists, flow.
- Why do different liquids flow at different rates? The rate of flow depends on how strong the forces are between the different molecules that make up a liquid, the size of the molecules and the temperature of the liquid.
Review all new words and write them on the class Word Wall. Finally, let students know that the next lesson will explore how temperature affects viscosity in liquids.
Fluid: Is a liquid or a gas. Fluids can flow because the molecules that make them up can slide past one another.
Viscosity: Viscosity is a measure of how much a fluid (liquid or gas) resists or does not allow it to flow. A liquid with high viscosity is honey which does not flow easily whereas a liquid like water flows easily, so it has low viscosity.
