Atom Frenzy – Lesson 3 – ‘Seeing’ Atoms and Molecules
With the aid of digital technologies, students will venture into the miniscule world of atoms by exploring Brownian motion and special microscopes to ‘see’ evidence of atoms. They will also act out water molecules bumping each other and into larger particles causing their movement.
1. Introduction: Introduce Lesson 3 by reviewing learnings from the previous lessons and introduce the Lesson 3 learning intentions using the Atom Frenzy Lesson 3 PowerPoint.
2. Teacher-led discussion: The search for evidence of atoms: Discuss how scientists search for evidence by looking for patterns and relationships.
3. Video Discussion: Evidence of Atoms – Brownian Motion: Outline the work of botanist Robert Brown and his connection to the naming of many Western Australian plants and his discovery of Brownian motion.
4. Activity (whole class): Acting out Brownian Motion: Students ‘act out’ Brownian motion by being water molecules jostling a beach ball that models pollen grains.
5. Video Discussion: More Evidence of Atoms: Then ‘fast forward’ about 70 years to 1905 when a young Albert Einstein correctly described Brownian motion as evidence of atoms that are always moving. Then uses a range of digital resources to investigate Brownian motion further: https://youtu.be/NHo6LTXdFns, https://youtu.be/gPMVaAnij88, and https://youtu.be/jLQ66ytMa9I
6. Video Discussion: Even More Evidence of Atoms – A boy and his atom: Complete the lesson by introducing electron microscopes by exploring the many online images of microstructures and introducing images of atoms. Finish by watching the short IBM video A boy and his atom: The world’s smallest movie on YouTube: https://www.youtube.com/watch?v=oSCX78-8-q0
7. Review and introduce the next lesson: Identify and review all new words and write them on the class Word Wall and introduce lesson 4.
The learning intentions for this lesson are that students will:
- know that scientists have direct evidence of atoms and molecules from modern electron microscopes
- explain that science involves searching for evidence and describing patterns and relationships
- This lesson again involves students ‘play acting’ being water molecules, requiring a suitable space to do so.
- Internet access will be needed as students will access online digital resources, either on their own, using a shared group computer or as a class through the teacher’s computer and whiteboard. It is recommended that teachers preview a range of electron microscope images.
- Large, inflated beach ball
- In our first lessons, we learnt that atoms are the building blocks of everything and that molecules are combined groups of atoms.
- Introduce the Lesson 3 learning intentions using the Atom Frenzy Lesson 3 PowerPoint.
Discuss some or all of the following questions with your students:
- What do detectives do? Find evidence that someone committed a crime.
- What is evidence? Things we see or other information that suggests or proves something was done by a person or thing.
Scientists are a little like detectives. They make predictions and search for evidence by looking for patterns and relationships.
What things did we learn in our introduction to atoms and molecules:
- Who can remember about how many types of atoms there are? About 100.
- What is an atom? An atom is the smallest part of a particular type of substance that exists- they are miniscule, being a ten-millionth of a millimetre across, they are made of protons, neutrons, and electrons.
- What is a molecule? One or more atoms joined together.
- How many different molecules there are? A huge number and some atoms like carbon can be put together an unlimited number of ways.
- What do we know about the size of atoms? They are miniscule.
- What is a microscope? A scientific instrument that allows us to see very small things using light that magnifies or enlarges an image.
- Can we see atoms using a normal microscope? No, they are much too small to be seen using light.
We start by telling students a very interesting story about Robert Brown, a scientist from Scotland who lived about 200 years ago. He loved microscopes and studying plants. In 1801, before British settlement in Western Australia, he visited the south coast of Western Australia where Albany now is and led an expedition to collect and name plants.
During his life, he achieved many things including finding out about microscopic plant cells. While studying plants under his microscope, he noticed that when tiny grains called pollen are in water they jiggle around. This is called Brownian motion.
The very brief video clip on the right demonstrates this:
Ask students:
- What do you think Robert Brown thought the reason for the pollen grains movement was? He may have thought the pollen grains were alive and moved like tiny fish or bugs of some sort.
- Do you think he was correct? Maybe/don’t know – (not enough evidence has been provided in the lesson yet).
- What do you think could be causing the pollen grains to jiggle around? Miniscule water molecules bumping into them.
He did not know at the time, but he had accidently stumbled across evidence that atoms and molecules are in constant motion and that when they get hotter, they move faster.
Students will now ‘act out’ Brownian motion by being water molecules and we will have a large beach ball that can be our grain of pollen.
Students will be liquid water. Ask students who can describe how water molecules in liquid water move:
- How far apart are they? Close together.
- Do they stay in fixed positions? No, they move all over the place.
- What happens when they get warmer? They speed up.
Students all stand in a close huddle, not too close, they still need to be able to ‘bump off’ one another and change direction.
Then they start moving like cold water. They are all moving slowly, gently bouncing off one another and gradually moving all over the place but staying close to the other molecules.
Now ask them to warm up a little. They all move a little faster, bumping into one another with more energy, still being very careful not to bump too hard, but staying reasonably close to their neighbouring atoms and still moving randomly around.
The ask everyone to stop for a moment.
Place the large beach ball in the middle of our huddle, and get students to do the activity again.
First they will be cold water.
- What does this mean about how fast we move? We move slowly, but still move randomly.
- What do we see happen to our beach ball? It is pushed backwards and forwards, causing it to slowly move all over the place.
Then ask students to warm up.
- How do we move now? Still randomly, but a little faster.
- How does the movement of the beach ball change? It is still pushed backwards and forwards, but more quickly, and it moves all over the place more quickly.
- Who can describe what happens in Brownian motion? The molecules of water push the pollen grains around randomly, just like we pushed the beach ball around randomly.
Now fast forward about 70 years to 1905. A young Albert Einstein correctly described Brownian motion as evidence of atoms that are always moving. He explained that it was water molecules bumping the pollen grains, just like bumper cars, that caused the pollen to move around.
Adding more heat causes the molecules to move faster. The movement results from the water molecules pushing the pollen grains around.
This can be seen in the following YouTube videos:
Over the years, normal light microscopes like those used by Robert Brown got better and better. Despite this, they will never be strong enough to see atoms and molecules that are thousands of times too small to see using light.
In the 1930s, scientists worked out that if they used a beam of electrons rather than light they would be able to ‘see’ much much smaller things. They called these new scientific instruments electron microscopes.
Then in 1986 special microscopes called Atomic Force Microscopes were invented by scientists that allow computers to ‘feel’ the surface of single atoms.
The very short IBM video: A boy and his atom: The world’s smallest movie is a movie made by scientists moving atoms one at a time.
IBM also made a short video explaining how they made the movie Moving Atoms: Making The World’s Smallest Movie. This is excellent teacher background but may also be of interest to students who are interested in how the movie was made.
Identify and review all new words and write them on the class Word Wall.
In addition, let students know that in the next lesson we will learn about the forces that hold atoms and molecules together.
Optional Extension Task
There are now several different types of electron microscopes.
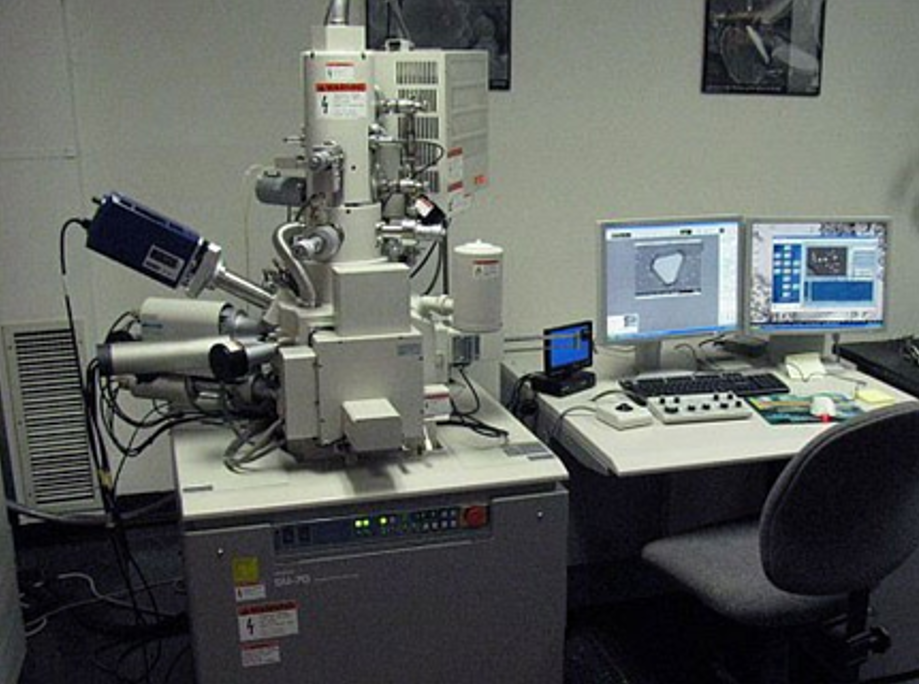
They allow us to ‘see’ things up to 1,000 times smaller than those that can be seen by the most powerful light microscope. The electron beam is changed into an image using light that we can see on a screen. Like detectives, scientists use these special microscopes to look at the way atoms are organised.
Electron microscopes are used in science research, industry, and medicine. It took many years and developments in electronics, computing, and engineering before electron microscopes could be used to see evidence of atoms and molecules. The images of atoms and molecules below show the tops of the atoms, like looking at a photograph of the tops of hills. Most of the time the atoms are in very neat lines, like fruit packed in a box.
If there is time, ask students to Google search: “electron microscope images of atoms” and draw some of the diagrams in their science journal.
Look at these and others.
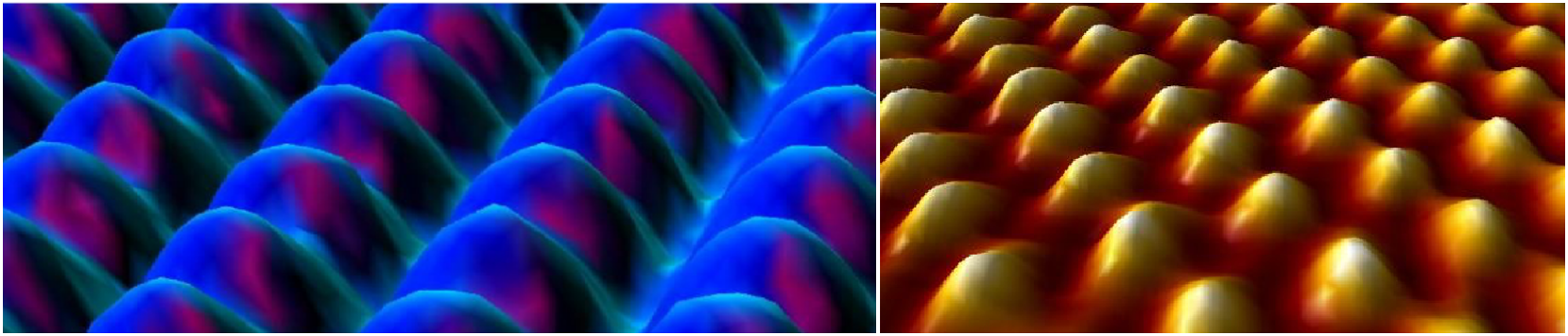
Ask students to share some of the images with their parents and search for more electron microscope images online.
Evidence: Information suggesting something exists, is true or is present.
Random movement: Movement in all directions or all over the place in a ‘higgledy piggeldy’ way.
Microscopic: Too small to be seen without the help of a microscope.
Brownian motion: Is the random movement of microscopic particles in air or a liquid caused by the continual bombardment from the surrounding molecules. It is evidence of atoms and molecules.
Microscope: Scientific instrument that uses light to see tiny things that cannot normally be seen by our eyes.
Electron microscope: Scientific instrument that uses a beam of electrons instead of light to see minute details that are too small to be seen using a light microscope
Bulletiness: Behaves like a bullet, has momentum, and can damage things.
