Atom Frenzy – Teacher Background
Einsteinian physics is based on two theories that emerged during the early 1900s and have been the major focus of physics and chemistry for a little over 100 years. The theories are special and general relativity that describe space, time and gravity at the cosmic scale, and quantum physics that describes the interactions of matter and radiation at scales down to the smallest subatomic particles.
The key ideas of these two theories are summarised in the following infographic:
- holists who thought that everything could be subdivided forever, and it would always be the same.
- atomists believed that everything is made of atoms.
In the second half of the 1800’s, chemists and physicists found more and more evidence for atoms. Despite this, the holists clung to their views, and to illustrate this, a famous chemistry textbook published in 1900 did not mention the word atom.
These battles were resolved in the early 1930s following a series of discoveries about the nature of matter. The story is summarised in the following audio clip and table.
The Amazing Story of the Atom
| Year | Discovery | Illustration |
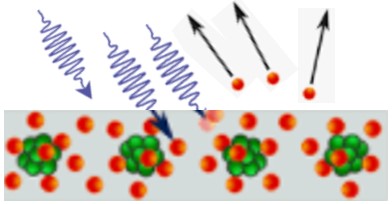
| 1887 | Photoelectric effect
Heinrich Hertz, Germany |
 |
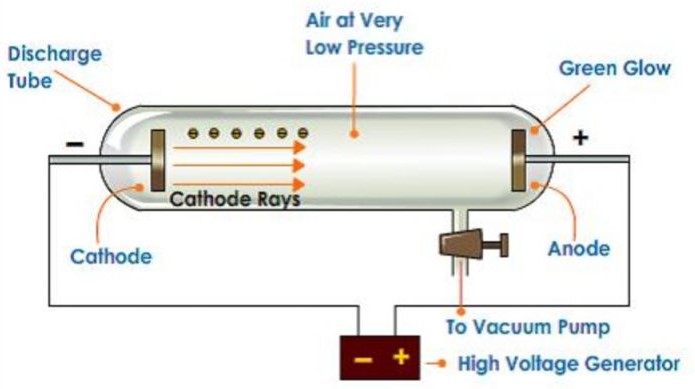
| 1897 | Electrons discovered
J.J. Thompson, England |
 |
| 1900 | Black body radiation and energy quanta
Max Planck, Germany |
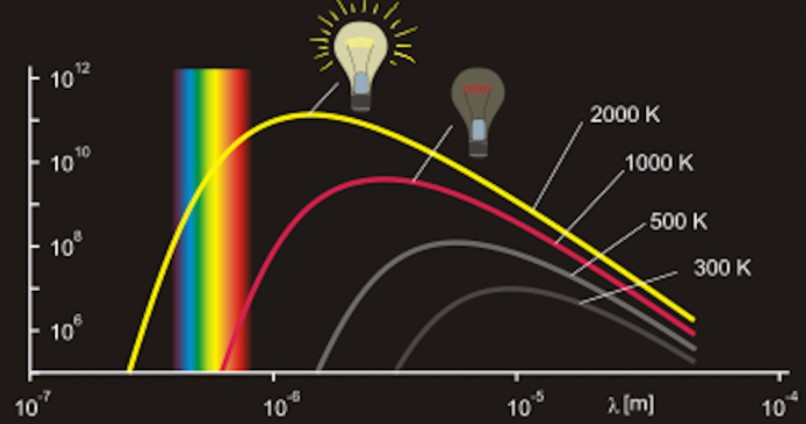 |
| 1905 | Light as photons and wave particle nature of light
Albert Einstein, Germany |
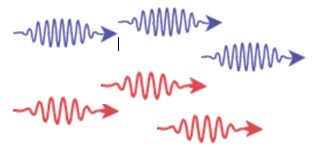 |
| 1913 | Bohr model of the atom
Niels Bohr, Austria |
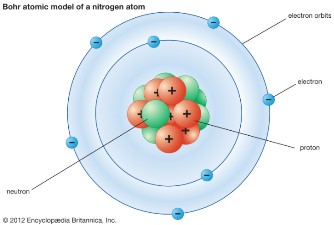 |
| 1917 | Nucleus and protons discovered
Lord Rutherford, New Zealand |
|
| 1924 | Wave-particle nature of matter
Lois de Broglie, France |
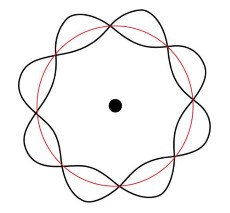 |
| 1926 | Wave equation model of the atom
Erwin Schrödinger, Austria |
 |
| 1927 | Uncertainy Principle
Werner Heisenberg, Germany |
 |
| 1932 | Neutrons
James Chadwick, England |
 |
Unlike relativity, which is primarily attributed to Albert Einstein, many scientists, including Einstein, contributed to the foundation of quantum mechanics. The new theory gradually gained acceptance through experimental verification between 1900 and 1930 and is now the basis of our modern understanding of atoms, molecules and matter, and provides the foundation of modern chemistry and physics.
Every child has the right to share our best understanding of physical reality
Foundation stones for future learning: These ideas form the foundation of future learning about the organisation and operation of the sub-microscopic world of atoms and molecules as students progress through school.
Familiarity with language and ideas in preparation for secondary school: We introduce the language and ideas of atoms, molecules and photons into the Year 3 Atom Frenzy and Hot Stuff modules and then consolidate this language, knowledge and understanding in Years 4, 5, and 6. We hope to have all primary school-age children familiar with the basic language and ideas of atoms and molecules so they will be familiar with and accepting of these concepts when they are introduced to them in secondary school.
Explanatory power: We also hope that having a preliminary understanding of the quantum nature of atoms and molecules will help students make the connections between what they see and experience in their ‘macroscopic world’ and explanation of these observations by appreciating what occurs at the sub-microscopic, atomic level. This will help them understand and appreciate the mysteries of the natural world.
The key ideas in introducing chemistry to Year 3 students are:
- Everything is made of atoms.
- Atoms join together to make molecules: little clusters from two to thousands of atoms, all stuck together by electrical forces similar to the forces we can feel between magnets or charged balloons.
- Atoms have a ‘fuzzy’ cloud of electrons surrounding an incredibly tiny blob of protons and neutrons called the nucleus: there is one electron for every proton. The simplest atom is number 1, hydrogen. It has one proton in its nucleus and one electron held by the force of attraction. One of the biggest atoms has 92 protons and 92 electrons in its fuzzy cloud: it is number 92 and called uranium. You might ask why we call it a cloud of electrons. The reason is that everything, including electrons and photons, combine two main properties: waviness, that makes them fuzzy, and bulletiness that makes them able to bump into and break things. The electron waviness makes them into a beautiful cloud pattern around each nucleus.
- Electron microscopes allow us to ‘see’ atoms: an important part of our background knowledge is modern images of atoms and molecules and even the world’s smallest movie called “A boy and his atom” where atoms are moved around to make a simple little 2-minute movie that children enjoy.
- Atoms and molecules constantly move because of heat. Einstein was the first to understand how heat is jiggling atoms. You can see the effect of jiggling atoms if you look at tiny particles floating in water through a microscope and see how they jump randomly around when a few extra atoms bounce off one side.
In Year 3 – Atom Frenzy we introduce atoms and molecules through role plays where children play four of the most common types of atom: hydrogen, carbon, nitrogen and oxygen. Through songs and games children become familiar with the concepts of simple molecules.
Atoms, photons, and electrons provide a means to explain and understand why things are the way they are. In Year 3 students apply this knowledge to explain the states and properties of matter and the nature of gases in the atmosphere.
The Einstein-First primary curriculum is designed to develop a scaffold of foundational concepts and language of modern science that will be developed in more depth in middle school. This will help ensure all students have a basic understanding of everything from climate to technology.
The four ‘main ideas’ introduced are: atoms and molecules; electrical forces hold everything together; miniscule things; and an introductory quantum model of atoms. It is these four main ideas we hope students will remember.
The following concept map summarises the key ideas introduced in this module and provides a concise narrative of the concepts presented:
If you are keen to have a little more background about the Periodic Table, the attached 11½-minute YouTube video: The Periodic Table: Crash Course Chemistry #4 is an excellent resource: https://youtu.be/0RRVV4Diomg
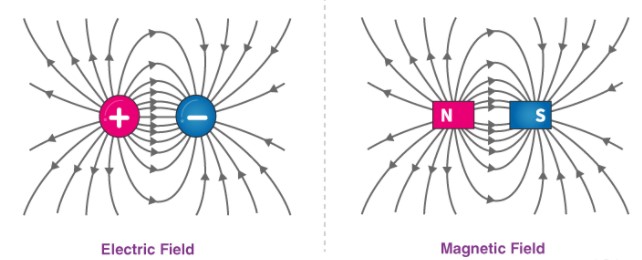
Source: https://byjus.com/physics/difference-between-electric-field-and-magnetic-field/
As explained above, matter is made of miniscule things called atoms. Each atom has an electron cloud around an even more miniscule nucleus. The nucleus is made of protons and neutrons. Protons attract electrons with an electric force because protons have an electric charge of +1, while electrons have a charge of -1. Protons and electrons attract each other because positive always attracts negative; this is called ‘opposite charges attract’. Positives repel positives and negatives repel negatives; this is called ‘like charges repel’. Neutrons have no charge, they are neutral.
Almost all forces we experience come from the electric forces that are made by electrons repelling one another. All the forces between atoms are electric forces. The air is made up of gases, mainly nitrogen and oxygen. The force of the wind we feel on a windy day is from the atoms that make up the gases bouncing off the atoms on the surface of your skin. They bounce because electrons repel each other.
Magnetism is also an electric force made when electrons move. The force between magnets result from the electric forces in atoms lining up to make force patterns that we can feel with other magnets or with things made from iron like nails.
The diagram below gives us an idea of the relative size of small and miniscule things:
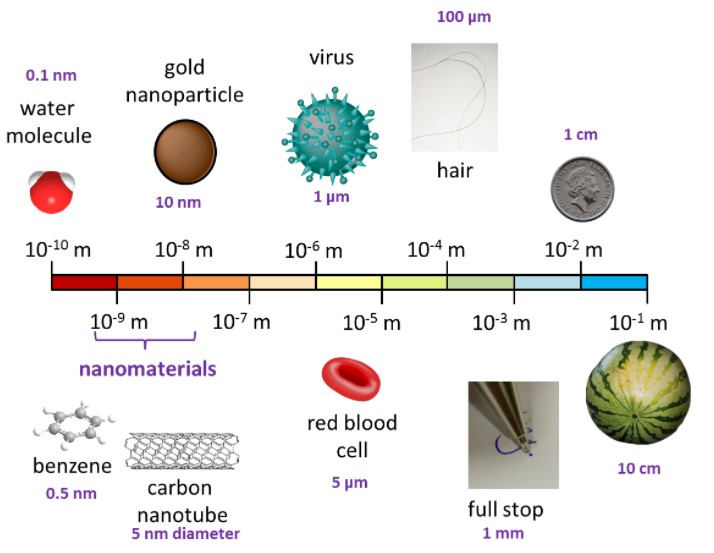
Nuclei are even tinier
An atom and its nucleus are much smaller still, with a typical atom being about 10-10 m diameter and it’s nucleus being about 100,000 times smaller at about 10-15 m diameter.
As students’ number knowledge develops in Years 4, 5 and 6, the idea of powers of 10 (e.g., 1,000 is 103 and one millionth is 10-6) will be introduced as a convenient way to show very small and very large numbers.
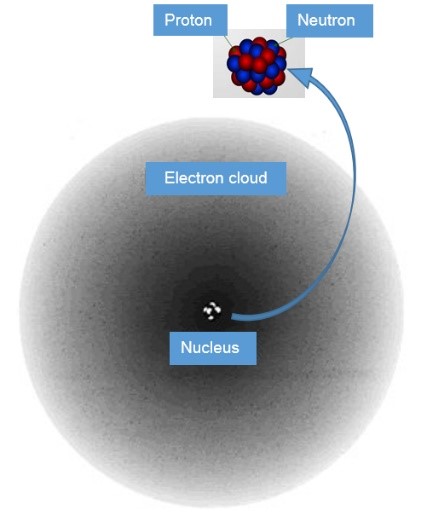
In 1926, Erwin Schrodinger developed the quantum mechanical or electron cloud model, where ‘clouds’ represent the location and movement of electrons around the nucleus.
Although this introductory model is very basic it can be developed further when students are introduced to ‘energy levels’ and ‘orbitals’ in Years 9 to 12.
For those who are familiar with chemistry, we avoid models that show electrons moving in fixed orbits around the nucleus, like planets moving around the Sun.
The goal is to introduce the vocabulary and concepts of atoms of atoms and molecules through play-based, highly engaging activities. In Year 3 we introduce just four key atoms and associated molecules through a class Reading Play. We aim for students to become familiar with hydrogen, carbon, nitrogen and oxygen that are elements number 1, 6, 7 and 8. These atoms and molecules are chosen because they are relevant, frequently used words and names that children hear frequently in news, cartoons, movies and everyday speech.
Teachers have the opportunity to extend the knowledge of atoms to the first 10 elements in a fun way through our ‘Atoms Song’. We do not expect children to learn the names of other elements, or any details about each, unless in an enrichment context.
By focusing on just four atoms, we hope students will remember the concept of matter made of atoms and atoms making the molecules that we breath and drink – O2, N2, H2O, and CO2.
When we introduce molecules, we need to answer the question why do atoms stick together to make molecules. This is easily done by using magnetised balls as toy atoms, because magnetism is the force made by moving electrons that is a key component of the forces between real atoms. Many fun activities allow students to explore the magnetic forces between their toy atoms, to see how attraction occurs for making molecules, and repulsion occurs to make atoms able to bounce off each other.
The above concepts provide the foundation for understanding heat, the jiggling of atoms, the three states of water -solid, liquid and gas – and also dissolving. We explore melting and freezing of water, dissolving sugar in water and the viscosity of honey and other household liquid foods via fun activities and simple experiments.
Developing a very basic understanding of atoms and molecules and the manner in which they interact with one another gives students the introductory knowledge needed to understand and explain their world. It is hoped Activities 5 to 8 relating to the freezing, melting and boiling of water, dissolving of sugar and viscosity of common household foods will introduce them to the wonders of science and experimentation.
The following videos provide additional insight into the structure of the atom:
The three states of matter most commonly encountered are solids, liquids and gases. There are other states including plasma, which is basically extremely hot gas. Plasma is so hot that all of the atoms’ electrons break away from the positive nucleus, resulting in a very hot mixture of electrons and nuclei (protons and neutrons). Atom Frenzy only introduces solids, liquids and gases.
The table summarises the main features of solids, liquids and gases.
| Solid | Liquid | Gas | |
| Illustration | 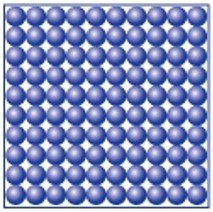 |
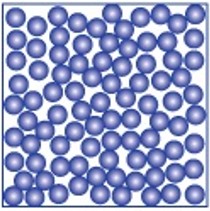 |
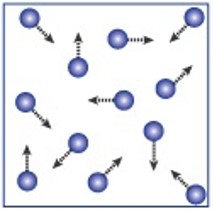 |
| Arrangement of atoms or molecules | In fixed positions packed closely together | Held closely together but are randomly arranged | Spaced out to fill the container they are in |
| Movement of atoms or molecules | Vibrate about their fixed position, and as they get hotter, they vibrate more | Move randomly around, and as they get hotter they travel faster | Move randomly around the container they are in |
| Shape and volume | Fixed shape and fixed volume | Takes container’s shape, fixed volume | No fixed shape or volume, fills container |
Source: The University of Waikato Te Whare Wananga o Waikato, www.sciencelearn.org.nz
Lesson 5 of Atom Frenzy introduces students to the states of water, namely ice (solid water), liquid water and water vapour (sometimes referred to as steam). In the lesson, the students also explore changes between these states: melting; freezing and boiling of water. The diagram provides a concise summary of the main ideas.
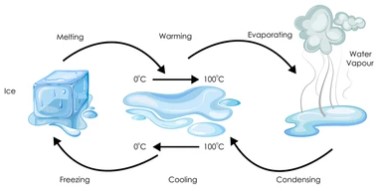
In ice, the water molecules are close together, held in a symmetrical pattern by electrical forces, and they vibrate backwards and forwards. When the ice absorbs heat energy from its surroundings, the water molecules increase the amount of vibration until the bonds holding the water molecules in their pattern break down and the ice melts. This happens at the melting point, which is normally 0°C.
The molecules are still close together, but they move randomly, mingling throughout the liquid. When more heat is absorbed, the water warms up and the temperature increases gradually from 0°C to 100°C. This results in the water molecules moving faster and faster until they ‘break out’ of the liquid’s surface. When the temperature reaches 100°C the water molecules separate, causing the water to boil.
Removing heat cools the water vapour to 100°C, at which point the water molecules reform into a liquid The liquid water then cools down until it reaches its freezing point of 0°C when they form ice. The following animation shows what happens when water freezes and helps explain why ice floats and snowflakes have hexagonal patterns.
Source: https://www.facebook.com/CSIROnews/videos/1162733547251890/?t=0
In this model, water or H2O, is a miniscule bent molecule made of a central oxygen atom (red) and two hydrogen atoms (white).
When water is a liquid, the molecules jiggle past each other but are quite ‘sticky’ due to the electrical forces between the oxygen and hydrogen atoms of different water molecules. As the temperature cools, water molecules slow down so the forces between the molecules now become very orderly. Eventually the water molecules no longer slide past each other, and the water turns to ice.
This animation helps us visualise and explain why ice forms a thin crust on top of water and then becomes solid from the top down. It also helps explain why ice floats. As the simulation shows, the ordered water molecules in a solid take up more volume than for the same amount of water molecules in the liquid state. Being less dense, the ice floats. You can also see that the ordering has hexagonal symmetry. When water vapour turns to ice as in snow crystal formation, this same ordering helps give the snowflake its characteristic hexagonal shape.

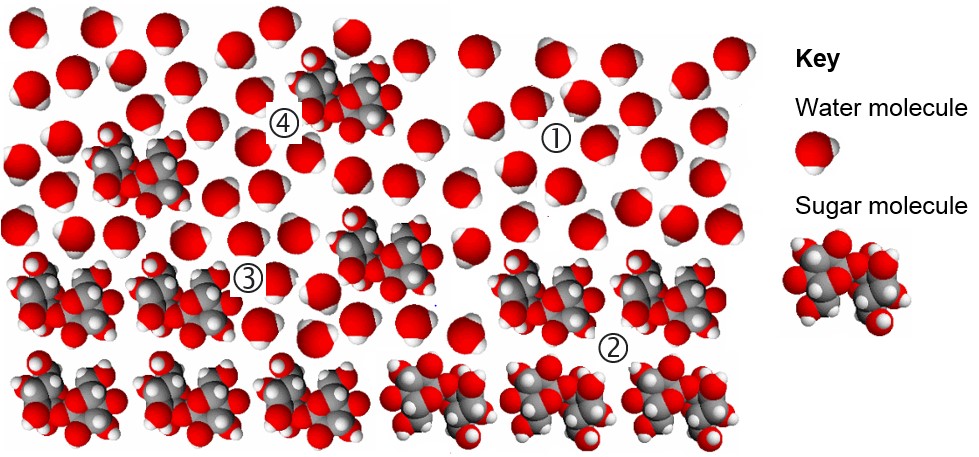
Students explore sugar dissolving in water in Lesson 6. Sugar crystals are made of individual sugar (sucrose) molecules (C12H22O11) held together by weak electrical forces. When sugar and water (H2O) are mixed, the water molecules move randomly and collide with the sugar molecules at the surface of each sugar crystal. The hotter the water, the more energy the water molecules have and the faster they move, allowing them to ‘break’ the sugar molecules away from the sugar crystal more readily. This is why sugar dissolves faster in hot water than it does in cold water.
In this digital animation, the green sphere represents a sugar molecule being ‘carried around’ by surrounding moving water molecules:
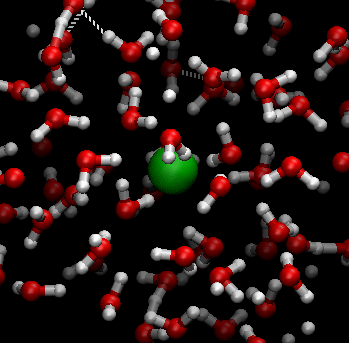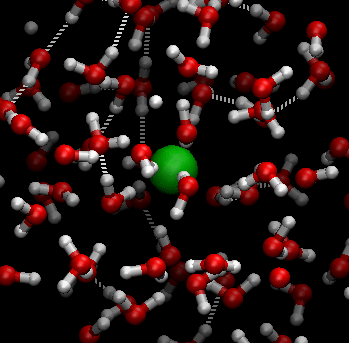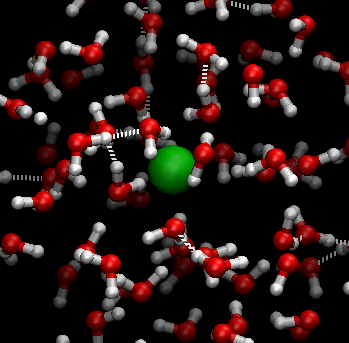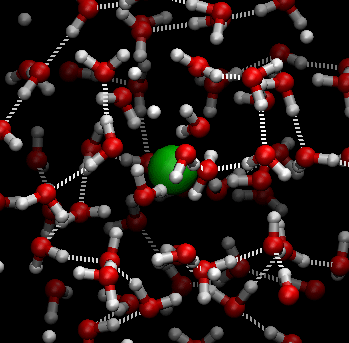
The following diagram also illustrates this process.
- The molecules in liquid water are close together and move randomly. They are ‘bent’, which means their shape has quite strong electric forces associated with them. The amount of heat energy they contain determines their speed. The hotter they are, the faster they move.
- Large sugar molecules are held together in the sugar crystal by weak electric forces.
- The moving water molecules in liquid water collide with the large sugar molecules at the edge of the sugar crystal, loosening the bonds holding them in the crystal until they ‘break away’.
- Large sugar molecules are then ‘suspended’ amongst and held by the water molecules once they have ‘broken away from’ the sugar crystal. There are weak electric forces between the ‘bent’ water molecules and the large sugar molecules.
Students investigate viscosity in Lessons 7 and 8. Technically, viscosity is a measure of its resistance to flow caused by frictional or ‘drag’ forces between molecules that make up the liquid. These frictional forces result from electrical forces between the molecules or layers of molecules and depend on several factors including their shape and size. A liquid with large viscosity does not flow easily because there is a lot of internal friction between its molecules whereas one with low viscosity flows easily because there is very little internal friction.
For the purposes of introducing the language and ideas about viscosity to Year 3 students, it is acceptable to relate viscosity to the stickiness, runniness or thickness of liquids.