Atom Frenzy – Lesson 6 – Sweet Water
Students act out dissolving – water molecules colliding with molecules in a crystal, dragging out molecules one at a time. Then, working in groups, they conduct an investigation of dissolving sugar. Each use cups of iced water, cool tap water and hot-tap water. They explain their observations by using their knowledge of forces between molecules and that heat is the vibration of molecules.
1. Introduction: Review key learnings about atoms and molecules from earlier lessons and present the learning intentions using the Atom Frenzy Lesson 6 PowerPoint.
2. Discussion – Sugar: Introduce students to the importance of sugars in our diet to produce energy for us to live.
3. Activity (small groups) – Dissolving sugar: Students then use the investigation planner to plan an investigation of sugar dissolving in cold, room temperature and warm water. They then work in their groups to conduct the investigation.
4. Discussion and Activity (small groups) – What happens when sugar dissolves in water: Teacher questioning to elicit understandings about how the shape of water molecules results in them having a positive and negative end that is attracted to the positive and negative parts of the larger sugar molecules. As they are moving, they can ‘break’ sugar molecules out of sugar crystals and then hold and carry them around in solution. Followed by watching a video showing the process. https://youtu.be/ZmWYG7qh0QA
5. Activity (whole class) – Acting out sugar dissolving: Students act out what has just been learnt about H2O and sugar molecules by ‘playing’ moving water molecules in a liquid which ‘break’ sugar molecules out of sugar crystals and carry them around in solution. Through questioning, use knowledge of atoms, molecules, and electric forces to explain how water molecules ‘pull’ sugar molecules apart.
6. Discussion – Sugar and food: Relate dissolving to students’ worlds by relating back to the importance of food, particularly glucose to fuel their bodies, by watching brief videos on how sugars such as glucose and sucrose are carried in solution in blood and the role they play in providing energy for humans and other living things. https://youtu.be/rX5aej5Sv4s, https://youtu.be/dhbJqDWtpMM
7. Review and introduce the next lesson: In their groups, students write down three main things learnt from today’s lesson and share these with their group members and the class. In addition, let students know that during the next two lessons we will explore how runny and sticky some liquids are, and we will relate this to the way the molecules in these liquids ‘stick together’.
The learning intentions for this lesson are that students will:
- know that dissolving is a bit like melting. Molecules jiggle from the solid surface of sugar and join the liquid atoms that are jiggling more.
- use models and analogies to explain how water dissolves sugar.
- 3 clear plastic cups or reusable tumblers per group
- 3 ‘pop’ sticks to stir with per group
- 3 sachets of sugar or plastic spoon and small cup of sugar per group
- Access to very cold water (ice bath), tap water and warm tap water
- 1 thermometer
- About 15 model water molecules (from Lesson 2)
- 5 or 6 empty small boxes (e.g. shoe boxes or paper boxes) with lids to be model sugar molecules
In this lesson, students are going to learn about how important chemistry and temperature are in our daily lives.
Introduce the learning intentions using the Atom Frenzy Lesson 6 PowerPoint.
Let students know they will learn about how solids dissolve in water and relate it to our daily lives.
- What happens to us when we get hungry? Has anyone heard of being “hangry”?
Have a brief discussion with the class about the importance of the food we eat, particularly sugars like glucose.
- Who can remind the class about why sugars such as glucose are essential to our bodies? They provide the fuel that allow our bodies to produce the energy we need to move, think, see, talk and so on.
Next, students will learn about how moving water molecules in liquid water collide with larger sugar molecules, causing them to ‘break away’ from their neighbouring sugar crystal sugar molecules which are ‘carried around’ in solution by the water molecules.
We recommend viewing the activity video to the right before reading the detailed activity instructions below.
In this activity students will explore how quickly sugar dissolves in cold, room temperature, and warm water.
In groups, students use their investigation planner (Worksheet – Investigating Sugar Dissolving) to plan their investigation (Note: the teacher may provide significant guidance if students are not yet familiar with the science investigation process.)
The following sequence of questions may be useful in planning this investigation:
- What question will we be investigating? How does the water temperature affect how quickly sugar dissolves?
- What do you predict will happen? Will all three cups dissolve at the same rate, will the cold cup dissolve fastest and the warm cup slowest, or will it be the other way around? Students write their prediction and give reasons why.
- What is the one thing we will change? Temperature.
- What will we measure? What would the change affect? How quickly the sugar disappears/dissolves, so we will measure the time it takes for the sugar to ‘disappear’ in each cup.
- What will we keep the same? Which variables will you control? The amount or volume of water, the speed we stir the sugar and water.
- How will you set up your investigation?
- What equipment will you need?
Once the planning is complete, ask students to work through their investigation.
To start, each group takes three clear plastic cups or reusable tumblers, and provide them with the following instructions:
- Fill one with cold water from the ice-water bucket – with no iceblocks
- Fill the second with cold tap water
- Fill the third with hot tap water. Warning: Remind students of the need to be very careful with hot water and its danger, and make sure that it is no hotter than 60°C.
- Measure and record the temperature of each cup of water.
- Place a sachet or level teaspoon of sugar in each cup and start your timer.
- Each group member gently stirs their water and sugar mixture using a ‘pop’ stick, being sure not to spill the mixture and that each group member stirs at about the same speed.
- Watch carefully what happens to the mixture and record the time that it takes for all the sugar to ‘disappear’ in each cup.
- In your planning sheet, record your observations (What you saw happen, the time it took each spoonful of sugar to completely dissolve and temperature of each cup.)
- Have one person clear away all equipment, wash the plastic cups, ‘pop’ sticks and thermometer.
Students will now use their knowledge of atoms and molecules and electric forces to explain how the water molecules pulled the sugar molecules apart and ‘carried’ them as a mixture of water and sugar.
- Does anyone know a long word starting with ‘s’ that we use to describe this sugar and water mixture? A solution.
- Who can name some other solutions? There are many, e.g. salt water etc.
- Yes, there are thousands and thousands of solutions. Many of them involve water, but others have a different liquid to dissolve things. Does anyone know of any other liquids that can make a solution? Some medicines use alcohol.
Now let us see if we can work out what happened when the sugar dissolved and why it is that water is so good at dissolving things. Ask groups to retrieve the water molecules that were made in Lesson 2. Ask them to place the molecules in the middle of a table so that everyone in the group can see. Ask students:
- What is the shape of a water molecule? Bent, it looks a bit like Mickey Mouse.
- Describe the end with the two H atoms? The protons from the H atoms are pointing out from the end.
- What charge will this side of the water molecule have then? Positive, because of the extra protons.
- What would be the charge at the other side of the H2O molecule? The extra electrons from the oxygen would make it negative.
On one of their H2O molecules have students write a ‘-’ sign on the O atom and two tiny ‘+’ signs on the two H atoms. Explain that the positive and negative parts of the water molecule are attracted to the positive and negative parts of the sugar molecules.
In this activity students act out what we have just learned about by using H2O molecules (represented by students) and sugar molecules (represented by empty shoe/paper boxes).
Students are going to pretend they are dancing water molecules. Their body will represent the larger O atom and they will hold two paper plates with a big H on each to represent the two H atoms.
- Where will your positive side be? The front due to the two H atoms.
- What about your negative side? The body/back representing the O atom.
- Who can tell me what the six empty paper boxes represent? Larger sugar molecules.
- Now, remember how water molecules behave when they are a liquid. Who can describe how they move? Randomly, when hotter they move faster, but are still close together.
The following gif shows the random movement of water molecules in a liquid, including when a few molecules turn into water vapour by escaping the liquid surface: https://www.chem.purdue.edu/gchelp/liquids/liqintst.gif
Ask students to write a brief sentence on the back of their Investigation Planner describing how molecules move in liquid water. (The water molecules are close together and move randomly throughout the water container. The bounce off one another and the walls of the container. As heat is added, they move faster).
Now we will look at another digital video animation of sugar dissolving in water:
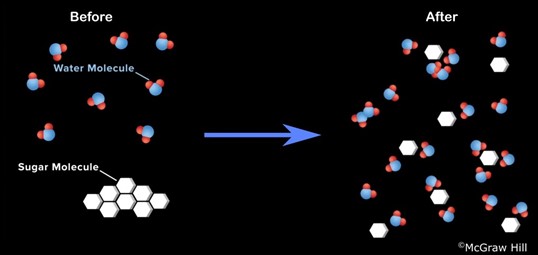
- What do the large white hexagonal shapes represent? Large sugar molecules.
- What shape are the water molecules? Bent.
- What attracts the water molecules to the sugar molecules? Electrical forces of attraction between the negative and positive parts of the molecules.
- Who can describe what the animation shows us? It shows water molecules moving around in liquid water and the white hexagonal shapes represent our larger sugar molecules These are ‘broken away’ from the solid sugar crystal and ‘held by’ the water molecules.
Remind students that we must always remember models and simulations are just that. They are not the real thing. They just give us a fairly accurate way of visualising what we believe is happening at the miniscule level of atoms and molecules.
Now place the six empty shoe/paper boxes in an open space and get everyone to stand randomly around them. We are now going to simulate the sugar molecules breaking free from one another and dissolving.
Then, each student play-acts being a water molecule just like they did before. Ask between four or five students to break one ‘sugar molecule’ from the ‘sugar pile’ and carry it around in solution.
Video the activity for future reference.
Questions to elicit understanding:
- What did we discover about how the temperature of the water affected how easily the sugar molecules dissolved? Higher temperatures make the sugar dissolve faster.
- What was our evidence? We measured the time it takes for sugar to dissolve in hot, cold, and room temperature water.
- Why did warm water dissolve the sugar molecules quicker? The water molecules were moving faster, so they could take the sugar molecules into solution more easily and more quickly.
- Why did it take the colder water longer to dissolve the sugar? The water molecules were moving more slowly.
- What do you think would happen if we kept adding teaspoons full of sugar to the warm water? Eventually there would be too much sugar and there wouldn’t be enough water molecules to ‘carry’ them into solution.
Now we will return to a discussion of hungriness and being “hangry” to finish off the lesson.
Watch the 3-minute WonderGroveLearn video: What Does Sugar Do to Your Body?
Then watch the Science for Kids YouTube video: Where Does Sugar Come From?
In their groups, ask students to write down three main things they learnt from today’s lesson and then share these with their group members.
Then facilitate sharing with the whole class before introducing what student will learn about in the next lesson.
If you aren’t intending to assess students understanding of the extension material in Lessons 7 and 8, then prepare for the post-test containing Questions 1-6.
Optional Extension Task
This could be integrated as an English writing task.
Using the writing framework students are familiar with, ask them to write their own ‘Hangry’ story using the information they have learnt about how sugars dissolve and break up into simpler sugars (fructose and glucose), and how glucose is dissolved in our blood and carried throughout our bodies where it is used to produce energy.
These stories could be shared with parents.
Sugar: sweet tasting molecules of carbon, hydrogen and oxygen atoms that are made naturally in plants. The simplest sugar is glucose (C6H12O6). Other sugars include sucrose, fructose, and lactose.
Crystals: a form of substance which occurs when many atoms that gather together in a solid make neat patterns which typically have particular shapes.
Binding energy: Energy needed to hold the molecules together in the crystal.
Dissolving: when the molecules separate or break away from one another and are absorbed by a liquid such as water and disappear from the solid. When sugar molecules break away from a sugar crystal and are ‘carried around’ by the water molecules, they disappear into the liquid. This is an example of dissolving.
Solution: A mixture of the molecules made up of dissolved molecules and the molecules doing the dissolving. In our activity, it is the mixture of the sugar and water molecules.
