Hot Stuff – Teacher Background
On this page you will find a brief description of the background knowledge required to teach the Year 3 – Hot Stuff module.
In the introduction of the Atom Frenzy Year 3 chemistry module, we learnt that for much of the 19th century there was a battle of ideas between two groups of scientists. The holists thought that matter could be subdivided forever, and it would always be the same, while the atomists believed that matter is made of an indivisible unit called atoms. During this time, scientists also believed that heat was a ‘fluid’ called ‘caloric’ that always flowed from hotter to colder objects.
In 1905, when Albert Einstein was aged just 26, he published a short paper that explained how Brownian motion could be explained by analysing the motion of atoms and molecules. This helped scientists understand the nature of heat. Based on Einstein’s insights, and the work of other scientists during the first three decades of the 20th century, it became clear the atomists were correct and that heat is the energy associated with the movement of atoms and molecules.
Electrons were discovered in 1897 by English physicist J. J. Thompson, allowing the relationship between electricity and matter to be recognised. Shortly after, in 1905, Albert Einstein showed that light (and other electromagnetic radiation such as radio waves, infrared radiation, ultraviolet radiation and X-rays) are tiny ‘bundles of energy’ that he called ‘energy quanta’. These bundles of energy behave like particles and are the ‘force carriers’ of electromagnetic radiation. They were named ‘photons’ in 1926.
Photons are introduced in this Hot Stuff module to help us understand the role that visible light and infrared electromagnetic radiation play in the transfer of energy. Photons will be re-visited in the Year 5 Fantastic Photons module.
We use Nerf guns as the model light source (like a torch, the Sun, or a laser) and the Nerf gun bullets to represent the photons. As with any model used to teach science, we should always carefully explain the model, how it is similar to and different from the ‘real’ thing. This is explored in a brief activity video for Lesson 2 shown below:
In 1907, two years after his discovery of photons, Einstein proposed that vibrations that carry heat and sound through materials also come as minute ‘quantised’ bundles of vibrational energy. This idea was largely ignored until the early 1930s when Russian physicist Igor Tamm revisited the idea and named these bundles of vibrational energy ‘phonons’ based on the idea that these are particles of sound, analogous to how photons are particles of light.
Many technological advances of the last century and our increased appreciation of nature is based on our knowledge of how photons and electrons interact, and the understanding that photons turn into phonons, which constitute the vibrational energy we know as heat.
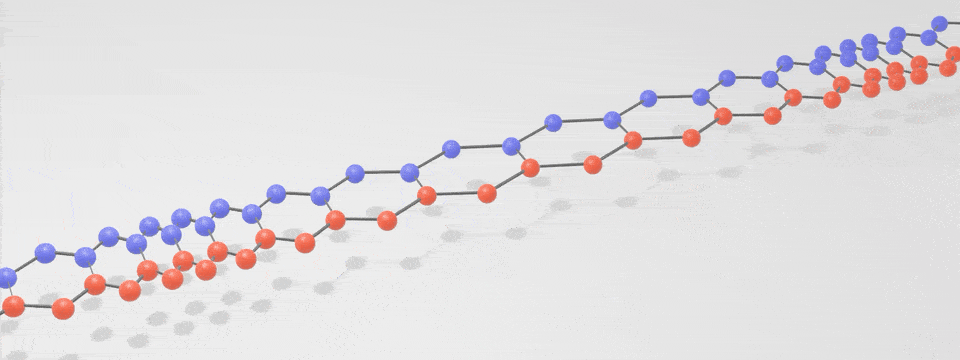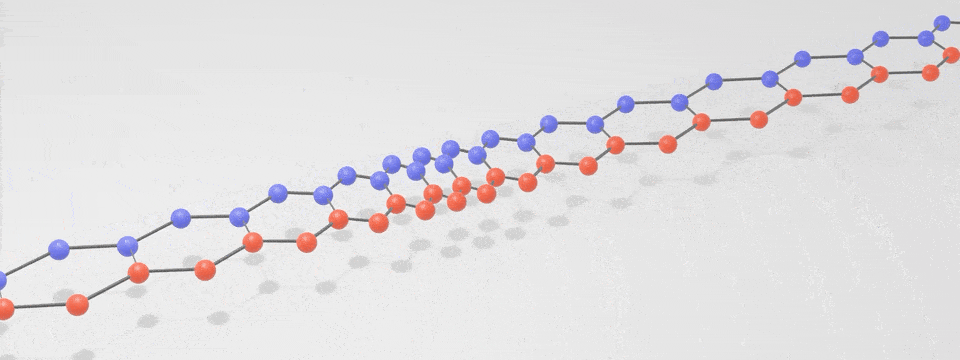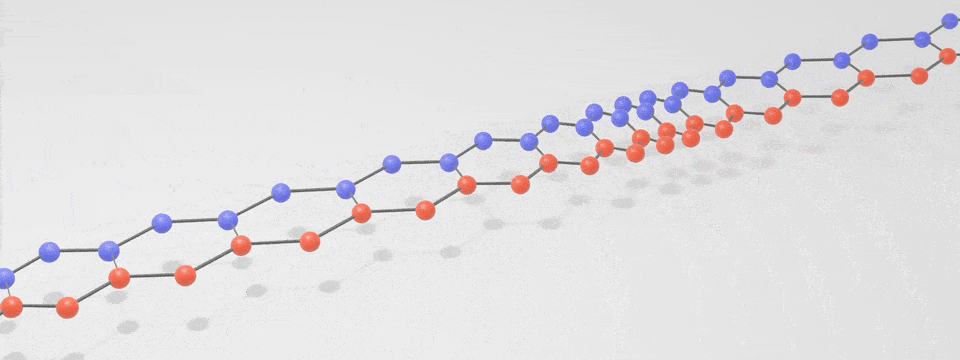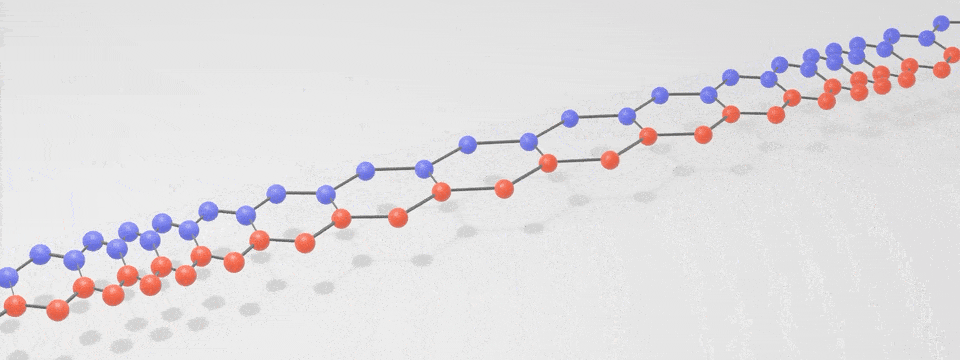
Just as there is a spectrum of light consisting of photons, with the different ‘colours’ having different vibration frequencies, so too is there a spectrum of phonons. At low frequencies these phonons are sound, and at very high frequencies they are heat. The following simplified phonon spectrum shows that both heat and sound are phonons.
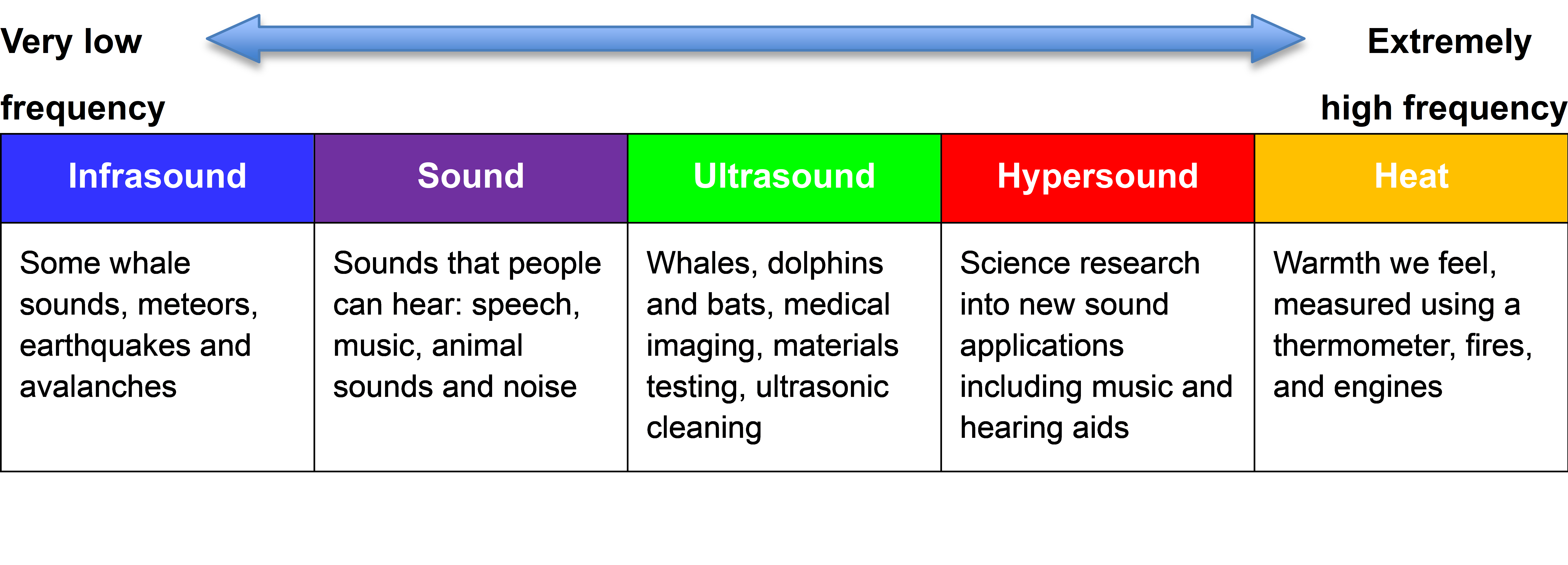
A ‘phonon’ is just a special word for a particle-like bundle of heat or sound energy. In most solids, the atoms and molecules are neatly arranged in patterns. They are held in place by electric forces that can both attract and repel like little springs. Heat is the energy of motion and vibration of atoms or molecules. When one atom is pushed or pulled, it sets off wave packets called phonons, like a tiny tsunami that travels through the solid. This is illustrated in the below digital visualisation from NIST:
A hot object is full of countless little phonon wave packets like the ripples in a swimming pool when many children are playing water games. Some phonons vibrate very fast, that is they have a high frequency. Others vibrate more slowly, particularly the big, slow waves like when someone does a ‘cannonball’ into the water!
For those who would like to learn more, the following one-page article provides a straight-forward description of phonons: https://news.mit.edu/2010/explained-phonons-0706
In practice, most materials are filled with a chaotic mix of phonons that have different frequencies traveling in random directions. They interfere with each other in a similar way to the messy movements of water waves in a choppy sea.
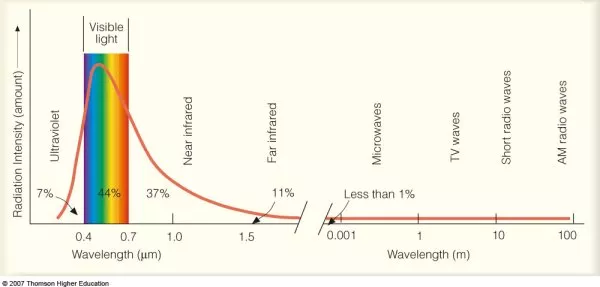
Almost all the heat energy on Earth comes as photons from the Sun. The Sun’s photons heat the land, causing the air to get hot and expand, and water to evaporate: these processes drive the different weather conditions we experience.
Analysis of the Solar spectrum shows there are different ‘types’ of photons. Roughly half (48%) are infrared photons which cause us to feel warm, a little less (44%) are visible light photons that allow us to see, and a much smaller amount (7%) are high-energy ultra-violet photons that cause sunburn and skin cancers.
Source: https://atmos.washington.edu/~hakim/101/radiation/
The Earth is also constantly losing heat energy because matter gives off infrared radiation, which is made up of infrared photons, which carry energy. Thanks to satellites, we can use infrared (night vision) cameras to ‘see’ how warm the Earth is from space. The imaging shows a glow with an average temperature of about 16 °C. But some of the Earth’s heat does not get back into space. Some is absorbed in the atmosphere because molecules of water, carbon dioxide, and methane, which each consist of only a few atoms, are very good at absorbing infrared photons. The energy that is reabsorbed helps keep the Earth warm enough for life, but too much of these gases causes global warming. This is explained more fully in the Year 6 Climate Science module.
The temperature of the Earth is set by the balance of heat coming in from the Sun and heat going back into space in the form of infrared photons. If the amount of heat escaping is reduced, the Earth gets hotter, just as you get hotter in bed if you add an extra blanket. The following brief European Space Agency video explains how this energy balance is achieved:
In addition to receiving almost all of our energy via photons from the Sun, small amounts of heat energy come from the hot centre of the Earth. Much of this heat is generated from changes to the nucleii of atoms caused by radioactive decay in the Earth’s centre as well as friction between the molten centre of the Earth as it slowly circulates beneath the solid Earth’s crust. Also, some is heat that remains from the Earth’s fiery birth billions of years ago. Volcanoes are places where we see this heat released when hot molten rock, dust, and gases escape from deep underground.
This heat energy from the very hot inner core and mantle of the Earth causes huge, but very slow, convection currents, like those you can see in hot pans of soup (miso soup is really good for seeing this!). Australia and the other continents are floating on top of this huge hot slowly churning liquid ball. The molten rock and iron churns around inside the Earth. As they churn, they drag the continents along at about 1 cm per year, causing earthquakes and volcanoes and pushing up mountains at the places where the continental plates slowly crash together.
Heat (or thermal energy) is the vibrational energy of the atoms and molecules that make up an object. Understanding heat is based on an appreciation of the structure and nature of matter which are summarised as follows:
- all matter is made of miniscule, sub-microscopic particles called atoms and molecules
- atoms and molecules are in constant motion
- atoms and molecules collide with one another and transfer their vibration energy to each other via phonons
- electric forces, like tiny springs, cause atoms to attract each other when far apart, and to repel each other when they are too close.
A concise background to heat is provided in the following 3½ minute video titled The Kinetic Molecular Theory:
Photons of different energy make up the electromagnetic spectrum. When photons are absorbed by an object like a road surface, their energy changes into phonons, which, as we saw above, are particle-like bundles of heat energy. In most solids, the atoms and molecules are neatly arranged in patterns. They are held in place by electric forces that can both attract and repel, like little springs. The heat energy can then be transferred by the phonons bouncing around inside all solids.
As the atoms jiggle around inside matter due to their heat energy, that jiggling motion can cause atoms to emit electromagnetic radiation in a process called blackbody radiation which we will learn more about in Year 5 Fantastic Photons, Year 6 Climate Science, and Year 9 Quantum World. This turns phonons back into photons and causes warm objects to emit infrared radiation, and very hot objects to glow with visible light.
This means that heat can be transferred between two distant objects when the phonons in one object turn into emitted photons that are then absorbed by another object and turned back into phonons. This is how most of the heat is transferred between the Sun and the Earth.
Conduction of heat is the transfer of thermal energy from one place to another via phonons. If a hot and a cold object are joined, then many of the phonons inside the hot object slowly transfer to the cold object, causing the cooler object to get warmer. Eventually the temperature of both become equal. This is because the average vibrational energy of all the phonons are the same in each object.
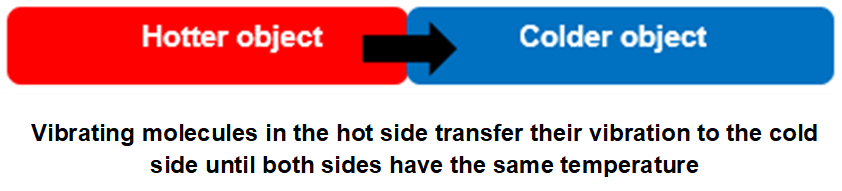
This is not the whole story of conduction though, as we see in metals. The free electrons found in most metals also contribute to heat transfer by conduction, by rapidly bouncing around in between atoms and bumping into them, distributing the vibrational energy as they go. We can feel this quite easily in our everyday experience with metals. For example, the handle of a metal spoon placed in a cup of hot tea will transfer heat from the hot liquid to your hand quite quickly. If a plastic or wooden spool is used, the heat is mainly transferred via phonons because almost all of the electrons are tightly held by the nucleus of each atom. This is illustrated nicely in the following video. Unfortunately, it does not use the language of phonons in explaining the transfer of vibrational energy from atom to atom.
The third way that heat can be transferred is called convection. This occurs in fluids (which is a collective noun which encompasses both liquids and gases) because the atoms and molecules are relatively free to move around. The following video animation explains this heat transfer process:
The atoms and molecules near a heat source gain energy, move faster, and bump into each other more, causing them to spread out and take up more space. This causes the heated fluid to become ‘lighter’, or more correctly less dense, and rise. The cooler, more dense atoms molecules then take their place. This ‘flow’ within the fluid is called a ‘convection current’ and is the main method of heat transfer in house heating, boiling water, weather systems, and ocean currents. Convection even occurs within the molten magma inside the Earth and in the interior of the Sun. Convection is explored in the Year 6 Climate Science module.
When a hot cup of tea is on a benchtop, heat flows from the cup and water to the surrounding air and benchtop through all of the mechanisms discussed above. But much of the heat is also lost in the process of evaporation which is caused by molecules vibrating so much that they ‘fly free’ of the liquid water and turn into gas, as was explained in the Atom Frenzy module.
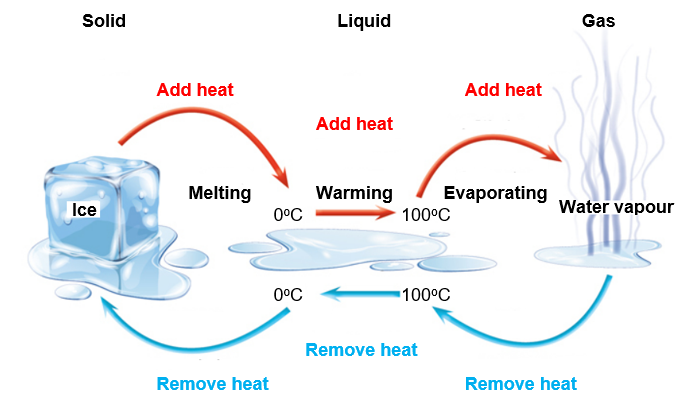
When heat energy is added to a solid such as ice its temperature increases until it starts to melt. The temperature then stays the same (its melting point) until the solid turns into a liquid. All of the added heat energy gets used up in overcoming the forces that hold the water molecules in their neat ice crystal structure, which means that the energy doesn’t get used to increase the temperacture. When all the ice is melted and the water molecules are now free to ‘mingle around’ in the liquid water, the added heat causes the water to get hotter and its temperature again increases. This is due to the heat energy now being changed to movement energy of the water molecules which travel faster and faster as they get hotter and hotter. As heat energy continues to be added, the water molecules can no longer stick close to one another. They spread out and form bubbles of water vapour which rise to the surface and escape into the atmosphere. This is explained in the following brief video:
Heat is essential for all living things, it creates the weather, and it makes mountains. Energy can come in little electromagnetic wave packets called photons which bring us the heat from the Sun and can turn into phonons in matter. Phonons, tiny little packets of vibrating atoms or molecules in solids and liquids, can move the heat around and make us feel warm.
In the Hot Stuff module, students learn that the Sun produces light and other radiation which travels outwards as tiny bundles of energy we call photons. When the photons collide with Earth they change into phonons and warm rocks and water. The phonons are ‘bundles’ of vibrational energy. The hotter an object is, the more phonons there are and the faster they vibrate. Models and analogies are used to explain photons and phonons, to explain how heat can be transferred in solids by conduction and learn that heat conducts via phonon vibrations travelling and creating vibrations at other places.
Almost 300 years ago (around 1740) Émilie du Châtelet (pictured top), a French scientist and mathematician, worked out that energy was some sort of ‘stuff’ that could be converted from one form to another. About 40 years later Scottish inventor James Watt (pictured below) showed how to measure energy and started selling steam engines that have the power of ten horses. Power is the name given to the rate we use energy. His steam engines turned heat energy from burning coal into the energy needed to lift buckets of coal out of mines.
Today nearly everyone buys energy in the form of electricity and converts it into different forms depending on how we want to use it.
We turn wind energy into electrical energy with giant wind turbines; electrical energy into lifting energy (it is called gravitational potential energy) in elevators and escalators; light energy into electrical energy with solar panels; and electrical energy into heat energy with heaters and stoves.
We send energy to communities via electrical wires, so we can buy and use it. We have electricity meters that measure how much energy we have used. We also buy energy in the form of gas that we can burn to make heat energy, and petrol that we can burn in car engines that turn the heat energy of burning into motion.


But the best cars are electric cars that turn the electric energy stored in batteries directly into the energy of motion (which physicists call kinetic energy).
Physicists worked out how to get energy out of the nucleus of atoms, a version of which is the process that powers the sun. Nuclear reactor power stations turn nuclear energy into heat which operates steam turbines to make electrical energy.
Everyone cares about energy because energy makes things happen: without energy nothing would happen!