Hot Stuff – Lesson 7 – Keeping hot things hot
Students learn about insulation. They then design and conduct a ‘fair’ investigation of the rates of cooling of warm objects with different coverings and use scientific understandings and language to explain different rates of heat transfer. In addition, there is an optional discussion with links to Biology and an extension science investigation.
Introduction: Explain that in this lesson students will learn about insulation and insulators and how we can help warm things stay warm and cold things stay cold. Introduce the learning intentions for this lesson using Lesson 7 of the Hot Stuff PowerPoint.
Acting like atoms – Modelling heat conduction in solids with insulation: Students model being a solid and how heat energy is transferred along the atoms and molecules by phonons; they discover what happens when insulation (a gap in the lines) is present.
Science investigation – Planning and discussion: The teacher leads the class in the design of an investigation to determine the rate of cooling of bottles of warm water with different coverings. (See the Keeping hot things hot investigation planning sheet)
Activity – Keeping things warm: Students work in groups to carry out the investigation and complete the investigation results sheet.
Discussion: Groups give feedback to the class about the effect of the different insulating coverings on the bottles and explain this using their knowledge of phonons and insulation.
Review: Review the main learning from the lesson and add to the class word wall.
Students will:
- know that insulators reduce the transfer of heat energy
- use scientific understandings to explain cooling and warming
- If not available in your science resources, collect about three dozen uniform glass or plastic 500 mL drink bottles.
- Obtain multiple different insulating materials (e.g. Old woolen sock/part of a sleeve, towel, cardboard, corrugated cardboard, layers of paper, neoprene fabric, bubble wrap etc.) for students to wrap around the bottles. Students may also use their own jumpers or scarves, etc.
For each group:
- 3 small empty clear plastic water bottles (labels removed) per group
- Insulating material to be wrapped around two bottles (see preparation notes)
- Rubber bands and/or tape
- If available, 3 student thermometer per group (otherwise 1 per group)
- Access to warm water (no hotter than 60°C)
- Timer per group (alternative: teacher calls time using an alarm)
Explain to students that in this lesson they will learn about how to keep things warm (optional extension: how to keep things cool so they do not warm up too quickly).
Introduce the learning intentions for this lesson using Lesson 7 of the Hot Stuff PowerPoint.
Remind students about Lesson 5 when the class modelled the transfer of heat energy in atoms and molecules via phonons. They are going to do this again, but with insulation now added.
As a class they represent a solid object made up of atoms and molecules.
Explain that each student represents an atom or molecule, and when they jostle backwards and forwards it represents the vibration of atoms and molecules when heat energy is added.
- What happens to the rate of vibration as more heat energy is added? The atoms and molecules will vibrate more rapidly.
- What about if heat energy is removed? They will vibrate less.
- How does the heat pass through the solid? Heat energy is passed through a solid by tiny ‘bundles’ of vibration energy we call phonons.
Students line up in three lines which are next to one another, but with a large gap (too far to reach across) part way along the lines.
The three students at the back of each line then gently jostle the students standing in front of them (without moving their feet), and they in turn gently jostle those in front of them until they reach the gap.
Video the simulation and watch it back with the students if the resources are available to do so.
Discuss the model with the students through questioning:
- What did we all represent when we were in our three lines? Atoms or molecules.
- Are we representing a solid or liquid? Solid.
- How do we know this? Atoms or molecules are moving backwards and forwards or vibrating around a fixed position.
- What did it represent when the students at the back started jostling those in front of them? Heating the back of the line.
- What happened when students in their lines had a gap – representing insulation – in front of them? They could not jostle the next students in front of them because of the gap; this is what happens when there is insulation: the vibrating phonons cannot pass on the heat energy through the insulation.
Students will now plan and conduct an investigation to model the effect of different types of insulation.
They will use two bottles of warm water with fabric and/or other coating; the third bottle with no covering will be their control.
Conducting a fair test will help to understand how having a covering will affect how well the bottles are insulated. The better insulator will keep a bottle warm by not letting heat energy be conducted through the insulation.
Remind students of the principle of ‘Cows Moo Softly’. What does this funny little saying stand for? (It is a useful scaffold or memory aid called a mnemonic that can be used to remind us how to plan a fair test):
- Cows: Change one thing (independent variable)
- Moo: Measure/Observe another thing (dependent variable) and
- Softly: keep the other things the Same (controlled variables).
Through questioning, students determine the best way to set up their investigation to ensure it is a ‘fair test’ and then complete their group’s Keeping hot things hot investigation planning sheet.
For students who are not familiar or confident in planning science investigations, remind them that when scientists undertake scientific investigations the first step is to ask the following two key questions:
- What are we going to investigate?
- What do we predict will happen?
Once this is clear, remind students that investigations involve measuring variables.
- What is a variable? Something that can be kept constant, changed, or measured in an experiment.
- What are some variables in our investigation? Temperature, time, covering, starting temperature, amount of water.
Variables can be changed (independent), measured or observed when they are affected by the change (dependent), or kept the same (controlled) in an investigation. Students need to identify the variables when planning an investigation to make it a fair test.
- In our investigation, what will we change?
- What will we measure?
- What will we keep the same?
- How often will we record the temperature of each bottle?
- How will we make sure that the temperature is not different at the top versus the bottom of the bottle? We stir it before taking the temperature.
Following this class discussion ask students to complete the investigation planner as either a whole class or in small groups of 3 or 4 students.
On the right you will find a demonstration video for this activity. We recommend viewing this video before proceeding with the text description below.
Working in their groups, students:
- assign roles and collect equipment required.
- set up their investigation.
- conduct the investigation according to their plan.
- record results in their science journal.
Alternatively, students could use a wide range of different insulating materials and collect data for all of these.
As an optional extension you may discuss or students may perform an additional investigation examining how insulation keeps ice water cold.
Once groups have finished their investigations, review the results as a class:
- What did your group find about the different insulation around the bottles and the effect that had on heat transfer?
- What can we say about insulation/insulators and how they work?
Review the main learning from the lesson and add to the class Word Wall.
Ensure that students complete the Post-test once you have finished Lesson 7
Optional Extension Task
Application to animals and humans
Use questioning and discussion to link students’ learning to everyday life:
- Do we always want to stop heat energy leaving our body? Not when we get too hot, instead we want to increase the amount of heat energy lost as losing extra heat energy has the effect of reducing our temperature making us feel cooler.
- What happens if we get too hot or too cold? Ask students to briefly discuss the diagram in their groups and write an explanation of how we cool down if we get too hot or warm up if we get too cold.
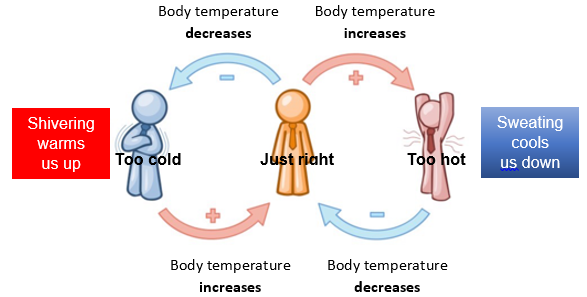
- How does sweating help cool us down? Heat energy from our bodies is used to evaporate the water in our sweat.
- How does shivering help to warm us up? Our muscles contract and expand rapidly which turns the chemicals in our muscles into heat which helps to raise our body temperature.
- Why do birds have feathers? Apart from to help them fly, they help keep their small bodies warm.
- What are birds often seen doing on cold mornings? Why do they do this? They fluff up their feathers – by fluffing up their feathers birds trap body heat close to them. Air is a good insulator and the air trapped within the feathers does not readily circulate.
- What about animals that have fur? Fur also traps air, which acts as insulation like a blanket or jacket. Some animals grow longer fur or can cause their fur to stand on end to trap more air when it’s cold.
- What are these reptiles doing? Lying in the sun to warm their bodies.


- Explain how this is happening? Photons from the sun are absorbed by the animal’s scales. The absorbed photons are converted to phonons which cause atoms and molecules under the animal’s skin to vibrate and move more rapidly which increases the animal’s body temperature.
- Apart from being modest, why is it useful to wear clothes in summer if we are trapping body heat? The environment may be a lot hotter than us and heat energy reaching our skin will move into our body to make us hotter! Thin fabric clothes will keep the direct heat energy from the sun off our skin and stop it from entering our body. Clothes help us to be ‘sun smart’ and protect us from harmful ultraviolet radiation.
- How do clothes affect the movement of heat energy out of your body? Clothes reduce the amount of heat lost from the skin; act as insulation; keeps the heat energy in the body.
Insulator: material that prevents or reduces the transfer of heat energy.
