Hot Stuff – Lesson 1 – Heat Vibrations
Through fun activities, discussion and digital resources, students model the vibration and movement of atoms and molecules in solids and liquids and use marbles and a clear plastic box to model them. Students also explore the melting of familiar solids placed in a hot water bath. They observe what happens and then answer the question: What is heat?
*Note, please ensure that students have completed the Student Attitude to Science questionnaire and the Hot Stuff knowledge Pre-test before starting Lesson 1.
Introduction: Introduce the topic, including providing a topic overview and present the learning intentions using Lesson 1 of the Hot Stuff PowerPoint.
Acting like atoms – Being solid, liquid, or gas: Students stand on a grid and simulate vibration and movement of atoms and molecules by moving back and forth whilst holding their grid position (solid), then mingling while staying close together (liquid) as the teacher “adds heat”, then moving quickly and randomly around, filling the ‘space’, for example the room they are in (gas).
Activity – Modelling solids, liquids, and gases: Working in groups, students use a small box of marbles/beads/polystyrene balls to model a solid, a liquid, and a gas. They can also use digital models to illustrate the differences between solids, liquids, and gases. Students draw each of their models and write a sentence describing each.
Activity – Melting solids: Working in groups, students construct aluminium foil boats and observe ice melting at room temperature and butter melting in a hot water bath.
Discussion: Ask each group to report observations to the class and explain what happened. Then ask groups to discuss and write their answer to the question: What is heat?
Review and introduce the next lesson: Review all new words and write them on the class Word Wall. In addition, let students know that in the next lesson we will learn that the Sun provides almost all the energy needed to produce heat on Earth.
Alternative Activity: Melting solids in the Sun: Working in groups, students place a piece of plastic wrap on the pavement, brick or tile that is in direct sunlight. Then they put a small piece of Copha, butter, and ice on the plastic in the direct sunlight.
Students will:
- know that heat is ‘bundles’ of vibrational energy called phonons
- explain that hotter objects have more phonons that vibrate faster
- use models and analogies to explain the effect of heat on atoms and molecules
- Review the Lesson 1 of the Hot Stuff PowerPoint
- The alternative activity will only be effective on a sunny day. Watch the weather forecast a few days before. The activity could be done on a different day before the lesson. Before the lesson identify an outside location in a sunny position for the activity.
- Identify a suitable location where students can stand in a 5X5 or 5X6 grid (grid to match student numbers – doesn’t have to be exact numbers).
- PrimaryConnections has useful teaching resources for teachers not familiar with word walls and science journals:
- Read our ‘Using models and analogies in teaching science’ guide.
- rectangular clear plastic container/picture frame (one per group)
- 30 x 1 cm diameter glass marbles, spherical craft beads, or expanded polystyrene spheres per group
- aluminium foil
- butter and crushed ice (in an insulated container)
- a medium sized shallow container
- marbles or small weights
Alternative activity:
- timer
- Copha (coconut oil shortening), butter, and ice per group
- infrared thermometer (optional)
We are starting an exciting topic called Hot Stuff. Students will explore some interesting things about heat and see how this helps us understand our world.
Introduce the Lesson 1 learning intentions using Lesson 1 of the Hot Stuff PowerPoint. Follow the directions under ‘Getting Started‘ if you haven’t done so already.
Before starting to explore heat, though, we need to remind students about atoms and molecules because we use our knowledge of atoms and molecules to learn about heat.
In this lesson we will use a number of models to explain the role atoms and molecules play in our understanding of heat. First, explain that the whole class will model heat energy in atoms and molecules.
As a class we represent a solid object made of atoms and molecules, like a block of ice. This solid will become a liquid and then return to a solid.
- In our model, what does each student represent? A (water) molecule.
- What does it represent when students jostle backwards and forwards? It represents the vibration of the molecules when they have heat energy.
- What about when they jostle more vigorously? As more heat energy is added, the atoms and molecules vibrate more. So, the hotter an object is, the faster they vibrate. If we remove heat energy, they will vibrate less.
A new idea we will learn is that heat energy is passed through a solid by vibrations in tiny ‘bundles’ which we call phonons. The hotter an object is, the more phonons there are.
To begin, students stand on the 5X5 or 5X6 grid (adjust for number of students, the last row does not have to be completely full).
Ask students to jostle backwards and forwards gently – while holding their grid position – to represent the vibration of atoms and molecules in a solid.
Warning: Instruct students to be mindful of their neighbouring ‘atoms and molecules’ by not moving too energetically. They might tread on their toes, or worse still, push them out of the ‘solid’!
The teacher holds up a sign/or tells students: ‘Heat Added’. The students now represent the solid heating up and vibrating a bit more vigorously.
The teacher again holds up a sign or tells students: ‘Heat Added’.
The students then represent the solid melting and becoming a liquid. This is modelled by each student moving a little more energetically and, instead of staying on their fixed grid position, they mingle slowly amongst each other, but still remain quite close together.
The teacher then holds up a second sign or tells students: ‘Heat Removed’. The students then ‘cool’ down a little, moving slower and getting closer together, until they return to being ‘a solid’ in their grid positions.
The teacher leads a discussion about the model and encourages students to answer the following questions:
- How are atoms and molecules arranged and how do they move in a solid? The atoms or molecules vibrate or move backwards and forwards around a fixed position.
- How are atoms and molecules arranged and how do they move in a liquid? They move more energetically, or a little faster, and randomly amongst one another, but they are still quite close together.
Consider recording a video of the students as they model a ‘solid’, showing the change to the ‘liquid’ and then back to a ‘solid’.
See a quick overview video of this activity on the right before reading through the detailed outline below.
Working in groups, students collect a small clear plastic box and partially fill it with marbles, craft beads, or polystyrene balls and use these to model a solid, a liquid, and a gas.
Students use what they learnt about solids, liquids, and gases in the class activity at the start of the lesson to then model the three states of matter using their marbles and box. This time, we will start with a gas and then “remove heat” so that we can model a liquid, then a solid.
- In our model, what do the marbles represent? Atoms or molecules.
- Describe the movement of the atoms or molecules in a gas? They fill their container, move randomly and very quickly, and bounce off the walls and other atoms or molecules.
- Describe what happens when the atoms and molecules ‘cool down’? They slow down and eventually are attracted together by electrical forces between the atoms and molecules, so they are quite close together, but still ‘mingle’ amongst one another.
- Then as the liquid is cooled down further, what happens? They slow down even further until they stop ‘mingling randomly’ amongst one another, and they settle in fixed positions, usually in some nice pattern. However, they continue to move, but they vibrate backwards and forwards around their fixed position in their grid. Solids are typically packed more tightly in a regular pattern.
They then have an opportunity to use their digital devices to examine a set of simple animations that show the differences between how the atoms and/or molecules move and are arranged in solids, liquids, and gases (or view the relevant slide in the PowerPoint). Students then draw their models representing a solid, liquid and gas.
Solid
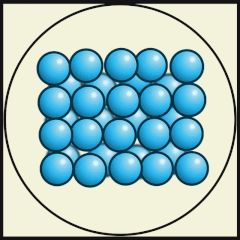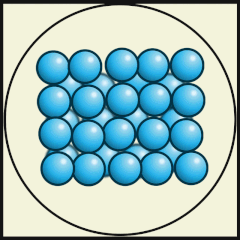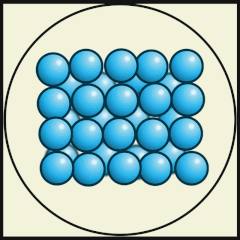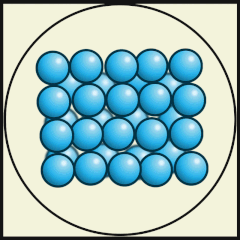
Liquid
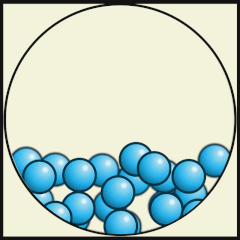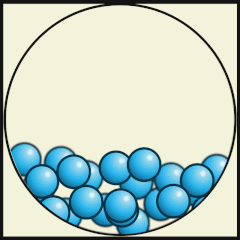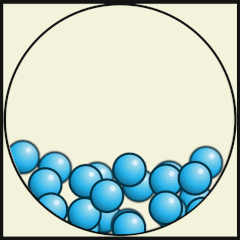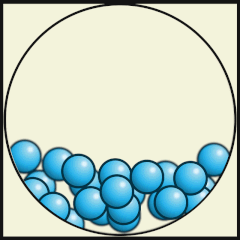
Gas
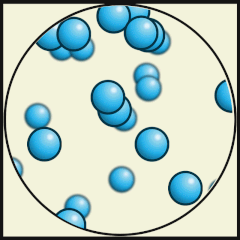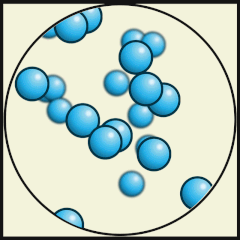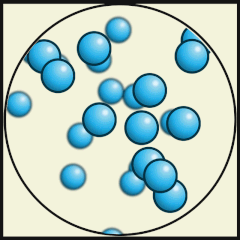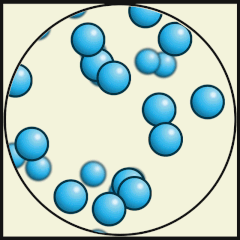
Source: Julio Miguel A Enriquez and Monica Muñoz (CC BY-SA 4-0)
See a quick overview video of this activity on the right before reading through the detailed outline below.
With students working in small groups, provide each group with a 10-15 cm square of aluminium foil, a cup, a few marbles and access to butter and a shallow container which will be filled with hot tap water.
Begin with a quick demonstration by placing a small piece of crushed ice and a dollop of butter onto a dish and leave it at the front of the class. Ask students what they think will happen to each substance if we leave them out at room temperature (the ice will melt but the butter won’t unless the room is very hot). Guide students to think about this in terms of how the tiny molecules are moving in each substance.
Then demonstrate how to make a foil boat by placing a cup in the middle of a foil square and folding up the sides. Then pinch down the sides until the walls are about 1-2 cm tall. Students should make sure that their boats will float by placing them in some cold water and then placing a few marbles into their boats. (You may like to integrate this activity with a separate arts and craft lesson in which students design and build a more sophisticated foil boat. Resources for building foil boats can easily be found on the internet.)
Instruct students to place a small dollop of butter in their boats, then collect some fresh hot tap water and float their boats on the surface of the hot water. Students should record their observations in their science journals.
Then return to the dish of ice and butter. Students should see that the ice has melted, but the butter is still solid.
- What is heat? Heat is the energy of atoms and molecules – the hotter something is, the faster the atoms and molecules jiggle about.
- What happens to the way atoms and molecules move when heat is added? Their speed increases, they vibrate more quickly.
- What are phonons? Little bundles or packets of jiggling energy that make up heat and sound.
- What happens when something melts? The atoms and molecules of a solid start jiggling so much that they break free from the electrical forces that hold them in place.
- Why did the ice melt at room temperature but the butter didn’t? The electrical forces that hold butter molecules in place in a solid are stronger than the forces that hold water molecules in place in ice. So it takes more jiggling or higher temperatures to break free.
Review all new words and start the Hot Stuff class Word Wall. Consider including key words learnt in the Atom Frenzy module including atoms, molecules, electrical forces, temperature, etc.
In addition, let students know that in the next lesson we will learn that the Sun provides almost all of the energy needed to produce heat on Earth.
Optional Extension Task
Another way to represent our model of heat energy in atoms and molecules is to use diagrams, digital images or video animations.
The kinetic theory of matter video: https://youtu.be/1Jtw8g795Us
The following simulator allows a range of variables to be explored. This simulation may be best completed as a whole class activity led by the teacher after he or she has practiced. Be sure to change the temperature to °C.
https://phet.colorado.edu/sims/html/states-of-matter/latest/states-of-matter_en.html
A second extension activity could be to take the video of the Kinaesthetic learning activity: Being solid, liquid, or gas and the students modelling solids, liquids, and gases using marbles in a plastic box. During a writing and/or digital technologies lesson, student could produce a script that could be recorded and integrated into the video to explain what is happening to the atoms and molecules in the solid, the liquid, and the gas.
After the Introduction you may wish to use the following activity instead of melting solids in a hot water bath.
Working in groups, place a piece of plastic wrap on some pavement, brick or tile that is in direct sunlight.
Then put a small piece of chocolate, Copha, butter and ice on the plastic in the direct sunlight.
If it is windy, add weights so the plastic does not blow away.
Observe what happens for the first 2 to 3 minutes.
Students then return later during the lesson to observe changes and record these changes in their science journal.
They may use a digital device to take photos of their investigation at the start and at the end of the lesson (alternatively, the teacher could take a photo of one set-up before and after for reference later).
Following the ‘modelling solids, liquids, and gases’ activity, return to the solids in the sun. Ask each group to record their observations about the small pieces of Copha, butter, and ice. If students took photos when setting up the activity earlier, ask them to take photos again to record the changes.
Ask students to work in their groups to report their observations and explain what happened.
- What happened to the Copha, butter, and ice cubes? Different solids melted or changed to a liquid, different solids melted more or less than others.
- Why did the tile get hot? Maybe the tile absorbs the sun’s photons and turns into bundles of vibrational or ‘jiggling’ energy.
- What is heat? Heat is the energy of atoms and molecules – the hotter something is, the faster the atoms and molecules move.
- What happens to the way atoms and molecules move when heat is added? Their speed increases, they vibrate more quickly. This is just like in their grid/moving activity and also their model using marbles in a box. This will help them explain why these changes happened.
Each group writes down what they think heat is. Groups share their ideas with the class; students and teacher come to an agreed definition as a class (the definition should include the movement of atoms/molecules).
The important points for students to understand are that:
- When sunlight strikes each solid and the tile or whatever they or on, photons that make up the light and other radiation are absorbed by the solids and surface.
- These photons turn into bundles of vibrational heat energy (which students will learn are called phonons in the next lesson), which results in the surface and solids getting warmer.
Danger:
- Never look directly at the sun and wear sun protection, particularly if the UV rating for the day is above 3.
- Be careful the tiles are not too hot to handle, they may burn students’ hands.
Atom*: Building blocks of all matter.
Molecule*: Two or more atoms held together by electric forces.
Vibration: Moving backwards and forwards, often about a fixed position.
Photons (if introduced): Individual ‘packets’ or ‘bundles’ of light or other electromagnetic radiation that carry energy and can travel through a vacuum.
Phonons: Bundles of vibrational heat energy in materials.
Solid: A solid is something which has a fixed shape and size or volume because its atoms and molecules are held closely together in fixed positions by strong electric forces. The atoms and molecules vibrate about their fixed positions.
Liquid: A liquid is something which has a fixed size or volume because the atoms and molecules are held closely together but its shape changes because its atoms and molecules are free to ‘mingle’ amongst one another because the the atoms and molecules are vibrating and jiggling too strongly for the forces between then to hold them in place.
Gas: A gas is something in which the atoms and molecules spread out and fill the container it is in because the atoms and molecules have vibrated and jiggled so strongly that they can break completely free from each other.
The following diagram provides a visual model of solids, liquids, and gases:
https://www.sciencelearn.org.nz/images/1839-three-states-of-matter
Model: Scientists use models to help understand the nature of the natural world. Models help us visualize or picture in our mind what something is like that is difficult or impossible to see. Models use familiar objects to represent these things that are difficult or impossible to see. A model can be a diagram or drawing such as the above example, a physical object (like a Nerf gun bullet), or a mathematical relationship (e.g., speed is distance travelled divided by time taken).
*Review from Atom Frenzy
